Celefill Automatic Filling Machines
Flexicon Aseptic aLiquid Filling
Industrial equipment
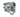
CellefillTM is a fully automated, turnkey system for filling vials in small batches with an integrated capping system.
The GMP-compliant system was developed by Flexicon Liquid Filling's aseptic filling and capping experts in collaboration with the specialists from Franz Ziel, a recognized global leader in development of sterile laboratory and production facilities.
Cellefill is a fully integrated solution for the biopharmaceutical industry that maximizes production capabilities through programmable recipes and provides the highest level of process control, resulting in increased production volumes for your products.
Cellefill optimizes the safety of each product batch with highly accurate peristaltic pump performance and integrated environmental monitoring during the manufacturing.
Our filling machines with the integrated capping and packaging systems enable you to maximize manufacturing of advanced therapy medicinal products (ATMP) including cell and gene therapies (CGT) products, small batch pharmaceuticals and biologics.
Features and Benefits
Celefill Automatic Filling Machines
Maximum manufacturing efficiency related to the controllable and programmable recipe, remote set-up and no format components for the entire range of vials

Fully integrated environmental monitoring system at strategically important facilities

Expert guidance and support throughout the project

Integrated aseptic filling system, and capping and packaging solution reduce operational risks and costs and improve total cost of ownership efficiency

Reduced risk of batch loss resulting from flexible in-process control including 100% weight check and zero waste start-up

Highly accurate and precision filling from microvolumes to 50 ml with an accuracy of less than ±1 % for most volumes

Manufacturing control for your biological and biopharmaceutical products, as well as compliance with GMP requirements

Celefill Automatic Filling Machines
Celefill Configuration
oRAB
Sterile protection of sensitive biological products, antibodies, cell proteins, or nucleic acid-based products. Class B / ISO 7 manufacturing room sterility.

Sterile protection of sensitive biological products, antibodies, cell proteins, or nucleic acid-based products. Class C / ISO 8 manufacturing room sterility.
iASP


iBIO
Hazardous biological agents, individual or live products requiring the highest level of cross-contamination control or BSL2 isolation. Class C / ISO 8 manufacturing room sterility.
Mon-Fri: 09.00 - 18.00
RK, Ust-Kamenogorsk, st. Tokhtarova, 51, off. 50
line of business
©2025. Alpha-Safety. All rights reserved.